Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: Patient-reported outcomes from a Phase 3 trial
Arthritis Care Res (Hoboken). 2014 Sep 3. doi: 10.1002/acr.22453. [Epub ahead of print]
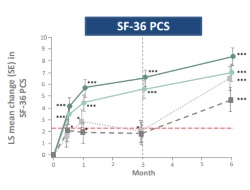
This paper presents patient-reported outcomes (PROs) form the ORAL Step trail, which assessed tofacitinib 5 mg or 10 mg twice daily, or placebo, in 399 patients with active RA, an inadequate response to at least one TNFi and receiving stable, background MTX.
PROs measured at month 3 included: Patient Global Assessment of Disease Activity (PtGA), pain, Health Assessment Questionnaire-Disability Index (HAQ-DI), Medical Outcomes Study (MOS) Short-Form Health Survey Version 2 (acute; SF-36), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), and MOS Sleep Scale.
With the exception of the MOS Sleep Scale, tofacitinib-treated patients reported significant, clinically meaningful improvements, versus placebo, in HRQoL. The short, 3-month, duration of this trial means ongoing extension studies are needed to address whether benefits observed in the short-term are maintained in the long term.