Sarilumab dans la polyarthrite rhumatoïde modérée ou sévère préalablement traitée: point de vue d'un groupe de revue de données factuelles d'une évaluation NICE à technologie unique
Pharmacoeconomics. 2018 Dec;36(12):1427-1437. doi: 10.1007/s40273-018-0677-7
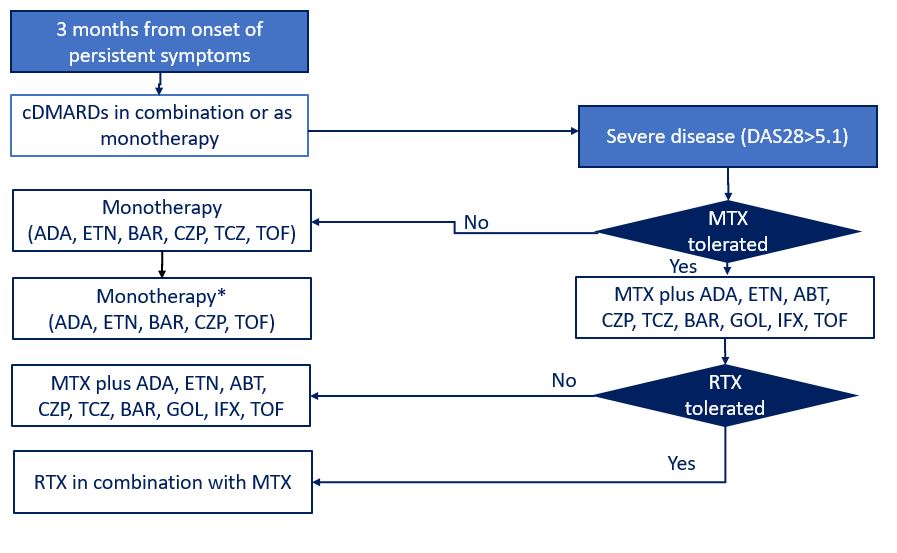
In this National Institute for Health and Care (NICE) single technology appraisal of sarilumab (SAR) monotherapy and combination therapy with methotrexate (MTX), SAR was considered to have similar efficacy to other bDMARDs for treating moderate-to-severe RA with inadequate response to cDMARDs or TNFis. SAR was also considered a cost-effective use of National Health Service (NHS) resources versus some or all of its comparators in most considered populations.NICE is an independent organisation responsible for providing national guidance on health technologies in England. For a drug to be recommended by NICE, its company must provide evidence to prove its clinical and cost-effectiveness. This evidence-based review reported the analysis of the evidence review group (ERG) for the single technology appraisal of SAR. In this analysis, the ERG concluded that the eligibility criteria were reasonable and consistent with the decision problem as outlined in the NICE scope, and the studies with SAR were of good quality. However, the ERG highlighted that the results should be treated with caution due to multiple problems with the study methods. SAR alone, or in combination with MTX, had similar efficacy for treating moderate-to-severe RA as other bDMARDs. SAR was considered cost-effective in most populations, except the TNFi-IR population and patients with moderate RA. To conclude, SAR mono- and combination therapy had comparable efficacy for treating moderate-to-severe RA as other bDMARDs recommended by NICE. NICE also considered SAR a cost-effective use of NHS resources, except in the TNFi-IR RTX-eligible population and for patients with moderate RA.