Secukinumab in Plaque Psoriasis — Results of Two Phase 3 Trials
N Engl J Med. 2014;371(4):326–338.
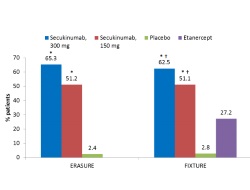
These two pivotal phase 3 studies in plaque psoriasis, FIXTURE and ERAUSRE, were sponsored by Novartis Pharmaceuticals. Secukinumab met all primary endpoints, PASI 75 response and the response of 0 or 1 on the modified investigator’s global assessment, as well as key secondary end points, at Week 12. Overall, secukinumab showed superiority to etanercept in improving moderate-to-severe plaque psoriasis symptoms in the FIXTURE study, and superiority over placebo in both studies. The incidence of adverse events during induction and the entire treatment period in the FIXRURE study were similar in the secukinumab and etanercept groups. These results validate interleukin-17A as an important therapeutic target in moderate-to-severe plaque psoriasis.
Secukinumab is the first therapy selectively targeting IL-17A to publish phase 3 results, and these data form part of the regulatory submissions for secukinumab in the EU and US, which were completed in 2013.1 Data from ongoing trials in arthritic conditions such as psoriatic arthritis and ankylosing spondylitis are also expected this year.1
1. Novartis Press Release. Novartis announces NEJM publication of two phase III studies demonstrating high efficacy of secukinumab (AIN457) in psoriasis patients. 2014. Available from: http://www.novartis.com/newsroom/media-releases/en/2014/1820056.shtml Accessed 12 Aug 2014.