Romatoid Artritli Hastalarda Tofacitinib’in Faz II, III ve Uzun-Dönem Uzatma Çalışmalarında Enfeksiyonun ve Tüm-Nedenlere-Bağlı Mortalitenin Analizi
Arthritis Rheumatol. 2014;66(11):2924–2937
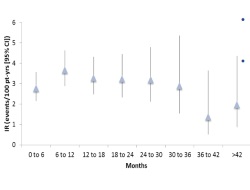
The overall incidence rate of serious infections was 3.09 events/100 patient-years (95% CI 2.73–3.49), which was stable over time, with pneumonia and skin and soft tissue infections being the most commonly reported. The rates of serious infection with tofacitinib in these studies were within the ranges reported from various large observational databases of patients receiving TNF-inhibitor therapies. Risk factors for serious infection included age, corticosteroid dose, diabetes, confirmed lymphocyte counts <0.5 x 10³/mm³, tofacitinib dose and, for herpes zoster infection, Asian ethnicity. Higher rates of herpes zoster infection were observed with tofacitinib treatment, although the majority of cases were mild-to-moderate. The all-cause mortality rate was 0.30 events/100 patient-years (95% CI 0.20–0.44), consistent with published rates in patients with active RA.
This paper provides important safety information regarding the use of tofacitinib in patients with RA, especially within the context of trends already seen in patients with active RA and rates already observed with biologic therapies for RA. However, as tofacitinib is a newer agent for the treatment of RA, vigilance for the emergence of any new safety signals or changes in trends of AE profiles remains imperative.