Aktif Romatoid Artritli Hastalarda Monoterapi Olarak Oral Selektif Bir JAK-3 İnhibitörü Olan Decernotinib’in Randomize, Çift-Kör, Plasebo-Kontrollü, On İki-Haftalık Bir Doz-Aralığı Çalışması
Arthritis Rheumatol. 2015;67(2):334–343.
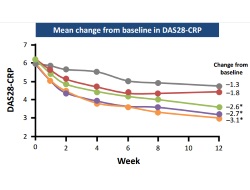
Four doses; 25 mg, 50 mg, 100 mg and 150 mg, were evaluated in this placebo-controlled study, with efficacy of each dose evaluated by ACR20 and DAS28-CRP at 12 weeks. Clinical signs and symptoms of RA were improved with the higher doses of decernotinib (50 mg, 100 mg and 150 mg) at the end of the study period, but infections and increases in liver transaminase and lipid levels were noted as potential safety signals.
Altogether, this data provides a rationale for further assessing the effect of decernotinib in patients with RA.